
Background:
Low-dose dasatinib was shown to be safe and effective in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP). There is no randomized clinical trials to compare the outcome with the standard-dose dasatinib. The aim of this study is to compare responses and outcome of patients with newly diagnosed CML-CP treated with frontline dasatinib 50 mg/day with those who received standard-dose dasatinib 100 mg/day.
Method:
We analyzed 233 patients with newly diagnosed CML-CP who were treated with low-dose dasatinib 50 mg/day (N=83) or standard-dose dasatinib 100 mg/day (N=150). Responses criteria were previously defined. Failure-free survival (FFS) was calculated from the start date of therapy to the dates of treatment discontinuation for any reason except of treatment-free remission; event-free survival (EFS), to the date of any of the events while on study as defined in the IRIS study; transformation-free survival (TFS), to the date of transformation to accelerated or blastic phases or death during study; overall survival (OS), to the date of death from any cause at any time or date of last follow-up. Patients on low-dose dasatinib who had suboptimal response by European LeukemiaNet criteria had an option to increase the dose to 100 mg/day. Propensity score analysis with 1:1 matching was performed with the nearest neighbor matching method using calipers of width equal to 0.2. Multiple imputation was performed to minimize the bias. Propensity scores were calculated with logistic regression from baseline covariates including age, spleen size by examination, white blood cell count, hemoglobin, platelet count, percentage of basophils, percentage of blasts in peripheral blood and bone marrow, the presence of clonal evolution, and Sokal risk classification to minimize difference.
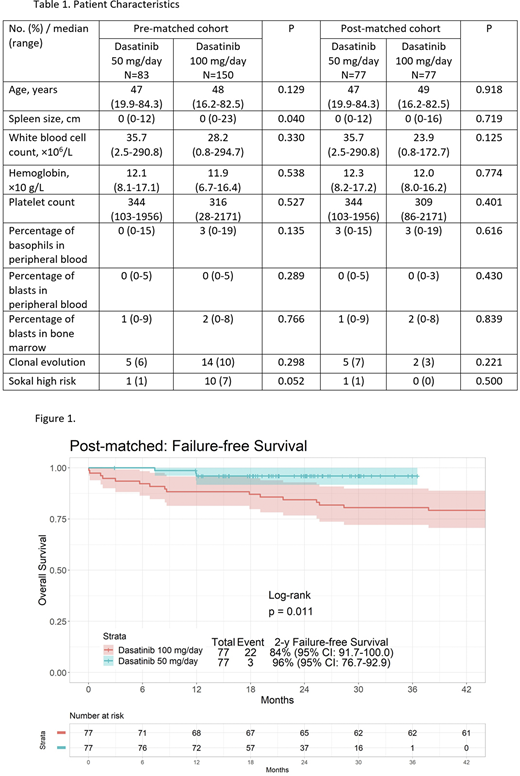
Results: The overall median follow-up was 84 months: 24 months and 120 months for low-dose and standard-dose, respectively. Propensity score matching identified 77 patients in each cohort without significant baseline difference (Table 1). The 12-month major molecular response (MMR) rates were 82% and 76% for low-dose and standard-dose groups, respectively (P=0.136). The cumulative incidence of molecular response (MR)4, MR4.5, and complete molecular response (CMR) rates within 1 year were higher in the low-dose dasatinib group compared with the standard-dose group (63% and 43%, 53% and 37%, and 24% and 11% for each)(P<0.001; P<0.001; P<0.001). The 2-year FFS rates were 96% and 84% in the low-dose dasatinib and standard-dose dasatinib, respectively (P=0.011) (Figure 1). The 2-year TFS rates were 100% and 100%, respectively (P=0.602); the 2-year EFS rates were 100% and 97%, respectively (P=0.157); the 2-year OS rates were 100% and 100%, respectively (P=0.602).
Conclusions: The low-dose dasatinib is at least as effective as standard-dose dasatinib with better drug exposure, resulting in better outcome.
Sasaki:Otsuka: Honoraria; Novartis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Pfizer Japan: Consultancy. Jabbour:Adaptive Biotechnologies: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding. Yilmaz:Daicho Sankyo: Research Funding; Pfizer: Research Funding; Pint Pharma: Honoraria. Bose:Promedior, Inc.: Research Funding; NS Pharma: Research Funding; Celgene Corporation: Honoraria, Research Funding; Incyte Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Blueprint Medicines Corporation: Honoraria, Research Funding; Kartos Therapeutics: Honoraria, Research Funding; CTI BioPharma: Honoraria, Research Funding; Constellation Pharmaceuticals: Research Funding; Astellas Pharmaceuticals: Research Funding; Pfizer, Inc.: Research Funding. Thompson:Adaptive Biotechnologies: Consultancy, Research Funding; AbbVie: Research Funding; Pharmacyclics: Research Funding; Janssen-Cilag: Honoraria; Genentech: Consultancy. Alvarado:Tolero Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; FibroGen: Research Funding; BerGenBio ASA: Research Funding; Jazz Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; Astex Pharmaceuticals: Research Funding; Daiichi-Sankyo: Research Funding. Jain:TG Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Precision Bioscienes: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Aprea Therapeutics: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Cellectis: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Garcia-Manero:Celgene: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Research Funding; Amphivena Therapeutics: Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Onconova: Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding; H3 Biomedicine: Research Funding. Burger:BeiGene, Gilead, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Gilead, Janssen, Novartis, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company: Other: Travel/accomodations/expenses, Speakers Bureau. Borthakur:BioTherix: Consultancy; Treadwell Therapeutics: Consultancy; Nkarta Therapeutics: Consultancy; BioLine Rx: Research Funding; Cyclacel: Research Funding; Novartis: Research Funding; Curio Science LLC: Consultancy; FTC Therapeutics: Consultancy; Argenx: Consultancy; PTC Therapeutics: Consultancy; BioLine Rx: Consultancy; BMS: Research Funding; AstraZeneca: Research Funding; Polaris: Research Funding; Xbiotech USA: Research Funding; Oncoceutics: Research Funding; Incyte: Research Funding; PTC Therapeutics: Research Funding; GSK: Research Funding; Jannsen: Research Funding; Abbvie: Research Funding. Pemmaraju:Pacylex Pharmaceuticals: Consultancy; Blueprint Medicines: Honoraria; Plexxikon: Research Funding; Stemline Therapeutics: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; DAVA Oncology: Honoraria; Samus Therapeutics: Research Funding; SagerStrong Foundation: Other: Grant Support; Affymetrix: Other: Grant Support, Research Funding; MustangBio: Honoraria; Incyte Corporation: Honoraria; Celgene: Honoraria; Daiichi Sankyo: Research Funding; LFB Biotechnologies: Honoraria; Novartis: Honoraria, Research Funding; Cellectis: Research Funding; Roche Diagnostics: Honoraria. Cortes:Daiichi Sankyo: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sun Pharma: Research Funding; BioPath Holdings: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Telios: Research Funding; Astellas: Research Funding; Amphivena Therapeutics: Research Funding; Arog: Research Funding; BiolineRx: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Immunogen: Research Funding; Merus: Research Funding. Kantarjian:Aptitute Health: Honoraria; BioAscend: Honoraria; Adaptive biotechnologies: Honoraria; BMS: Research Funding; Ascentage: Research Funding; Novartis: Honoraria, Research Funding; Jazz: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oxford Biomedical: Honoraria; Janssen: Honoraria; Sanofi: Research Funding; Pfizer: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Delta Fly: Honoraria; Daiichi-Sankyo: Honoraria, Research Funding; Immunogen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal